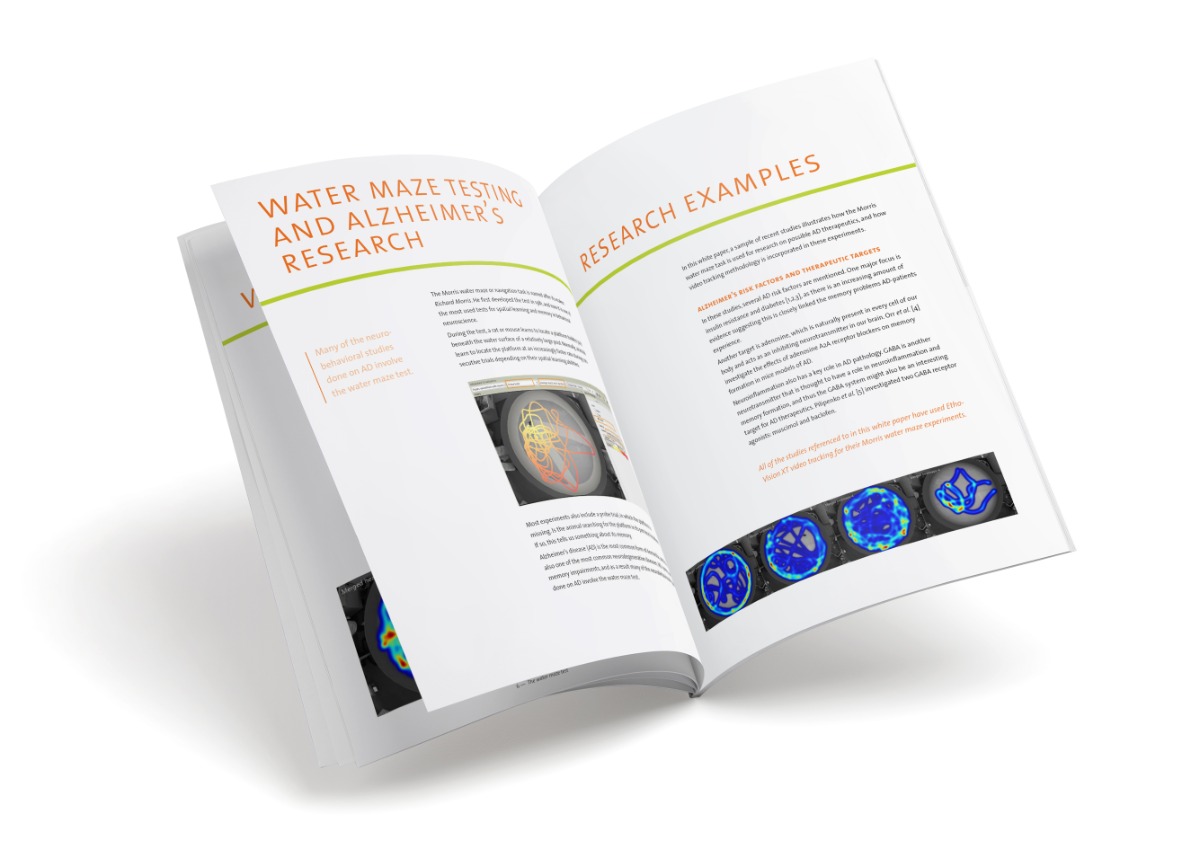
Tracking a wide variety of animals
Video tracking is used to track a widevariety of animal species in even more different test arenas. While one researcher wants to track insects of 1 mm on leaf discs, another one is interested in monkeys in a cage, or zebra fish in an aquarium. It will be clear that the settings created for detection of the tiny insect may not be suitable to detect the monkey, or the zebra fish.
Different setups require different detection methods
EthoVision XT tracking and analysis software has four methods to optimize the detection of your animals. Each method has its own benefits. This way the program offers possibilities to track many different animal species under very different circumstances. Below we summarize which method is most suited in which situation and give some examples of research carried out with these methods.

Gray scaling
Of the four detection methods, gray scaling is the most robust. This method identifies the animal by its gray scale value. This method is very suitable when the animal contrasts well with the background and there are no objects in the arena of the same size and gray scale as the animal. Furthermore, the background should not change, for example because bedding material is kicked around in a cage. Lavorato et al. [1] successfully used this method to track tadpoles in water. Also, Kloth et al. [2] used gray scaling to create 20 tracks simultaneously of aphids kept individually on small leaf disks.
Static subtraction
Gray scaling is not suitable if other objects in the arena have a similar color as the animal. If this is the case, for example because there is a novel object in the arena, tracking may be possible with static subtraction. In this method, a reference image of the background without animal is used. When your animal is present in the arena, EthoVision XT detects it by subtracting the reference image from the images with animal. Mathur et al. [3] used this method to track adult zebra fish in aquaria. This method works very well when you can create a reference image of your arenas without animals. But this is not always necessary. It is, however, necessary that the background remains constant throughout the experiment. When this is not possible, dynamic subtraction, as described below, is more suitable.
Dynamic subtraction
In dynamic subtraction, the background is updated regularly. So changes in lighting, or background, are corrected for. You can use this method, for example, when you track rats or mice in a cage that move bedding material around during the test. Also when the lighting changes during the test, dynamic subtraction may be what you need. This method is also very suitable when you cannot make a reference image of an arena without your animal. Ettrup et al. [4] used dynamic subtraction to track minipigs in pens of 2 x 1.5 m, probably because it would not be very practical to remove all minipigs from the pens to make reference images without animals. Also, Hammamieh, Rasha et al.[5] used this method to track mice in resident cages in which an iron mesh cage with an intruder mouse was placed.
Differencing
The detection method differencing is the most processor intensive one. For each pixel, EthoVision calculates the probability that its difference with the reference image is large enough, so that it is likely that it is part of the animal. When your animal is not well enough detected in all places of the arena with one of the other methods, try differencing. Differencing is especially suitable when there is a sharp light gradient in the arena, like a shadow, or when your animal has more than one color, like hooded rats. Assareh et al. [6] successfully used differencing to track Lister hooded rats in Perspex boxes.
The method that fits your needs
The research examples illustrate that EthoVision XT can be used successfully under very different circumstances, from aphids of 4 mm on leaf discs, to zebra fish in aquaria, rats or mice in home cages, and mini pigs in pens of 2 x 1.5 m. There is always a detection method fits exactly your research setup, even when tracking is challenging, for example because the background changes during the experiment, or when your animal has more than one color.
References
- Lavorato, M.; Bernabò, I.; Crescente, A.; Denoël, M.; Tripepi, S.; Brunelli, E. (2013). Endosulfan effects on rana dalmatina tadpoles: quantitative developmental and behavioural analysis. Archives of environmental contamination and toxicology, 64 (2), 253-262.
- Kloth, K.J.; ten Broeke, C.J.M.; Thoen, M.P.M.; Hanhart-van den Brink, M.; Wiegers, G.L.; Krips, O.E.; Noldus, L.P.; Dicke, M.; Jongsma, M.A. (2013).High-throughput phenotyping of plant resistance to aphids by automated video tracking. Plant Methods, 11 (4), doi: 10.1186/s13007-015-0044-z
- Mathur, Priya, Michael A. Berberoglu, and Su Guo., 2011 Preference for ethanol in zebrafish following a single exposure. Behavioural Brain Research, 217 (1), 128-133.
- Ettrup, K. S., Sørensen, J. C., Rodell, A., Alstrup, A. K. O., & Bjarkam, C. R. (2012). Hypothalamic deep brain stimulation influences autonomic and limbic circuitry involved in the regulation of aggression and cardiocerebrovascular control in the Göttingen minipig. Stereotactic and Functional Neurosurgery, 90 (5), 281-291.
- Hammamieh, R., Chakraborty, N., Lima, T., Meyerhoff, J., Gautam, A., Muhie, S., & Jett, M., 2012. Murine model of repeated exposures to conspecific trained aggressors simulates features of post-traumatic stress disorder. Behavioural Brain Research, 235, 55-66.
- Assareh, N., ElBatsh, M. M., Marsden, C. A., & Kendall, D. A. (2012). The effects of chronic administration of tranylcypromine and rimonabant on behaviour and protein expression in brain regions of the rat. Pharmacology Biochemistry and Behavior, 100 (3), 506-512.
Get the latest blog posts delivered to your inbox - every 15th of the month
more

Noldus Grant Assistance Program launched in North America
Grant funding is the lifeblood of academic research. Is successful funding the best path to securing future funding?
Furthering behavioral studies for understanding microbiota-brain insights
How and to what extend do microbes in the gut affect the brain and behavior? Discover how they do this kind of research at UCLA.